Dry Macular Degeneration
Symptoms, Treatment and Research
Dry Macular Degeneration is the most common form of age related macular degeneration (AMD). It is a retinal eye disease that affects what we see right in front of us. It often runs in families and in those over 65.
What is Dry Macular Degeneration?
Perhaps you have heard for the first time that you or your loved one has macular degeneration. Often times patients are given the diagnosis but then left on their own to find out what it means, how it progresses and what kind of vision loss is to be expected.
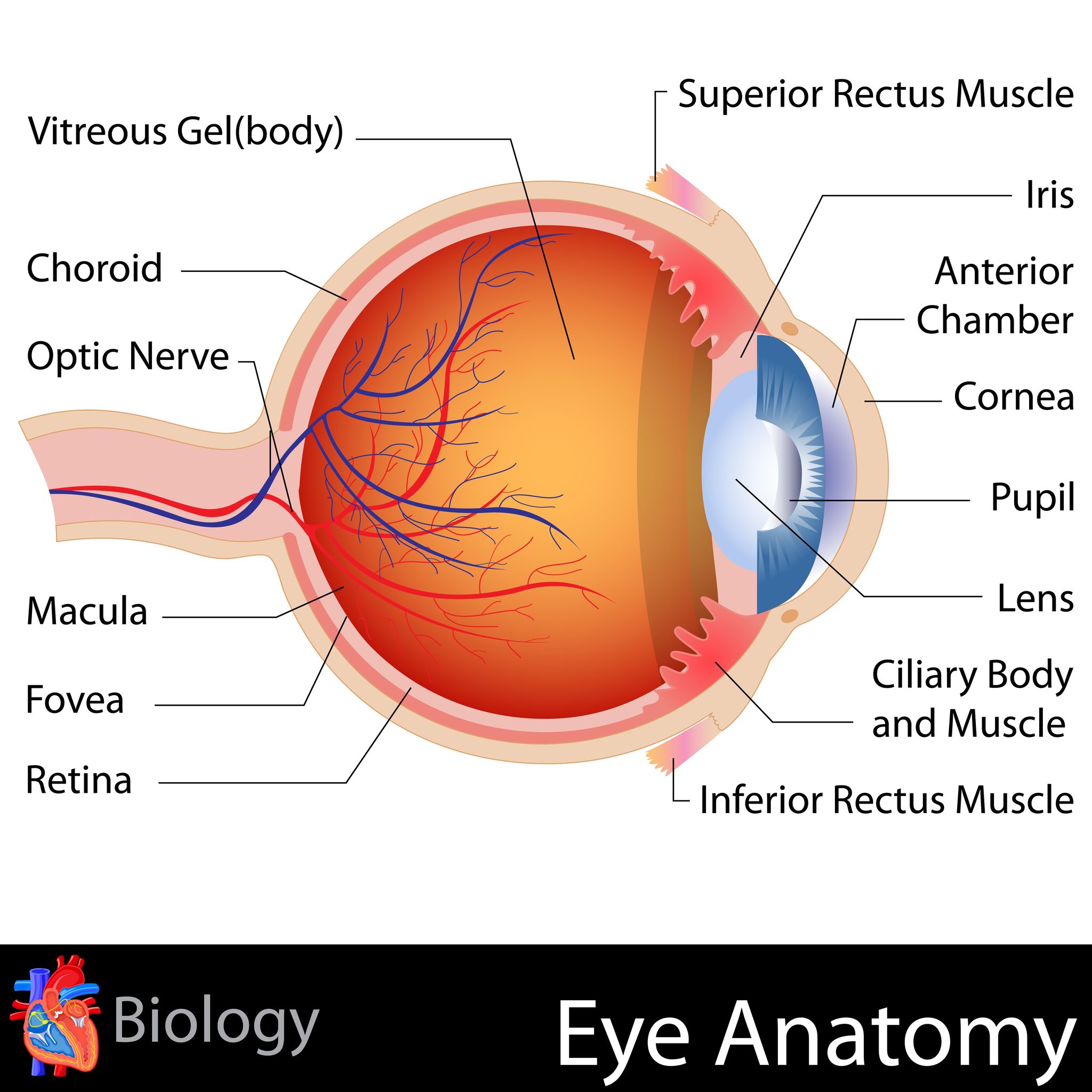
What is macular degeneration? It is a retinal eye disease specific to the macula - a tiny spot in the middle of the retina responsible for our detailed and straight ahead vision.
From this eye anatomy picture you can see where the macula is located - in the back of the eye in the center of the retina.
The macula provides us with our ability to see detail - such as reading a book or threading a needle. It also provides us with our central vision or straight ahead vision - like seeing a road sign or recognizing a face.
Dry Age Related Macular Degeneration
Dry age related macular degeneration (AMD) is given this name because typically it is a retinal condition that affects the senior population. The older a person is, the higher the risk he/she has for developing AMD. The dry form means that the eye has not developed fragile and leaking blood vessels under the macula which causes a more sudden and severe vision loss.
Other names for this macular disease is atrophic macular degeneration or non-neovascular AMD.
Dry Macular Degeneration Symptoms
Perhaps you've realized that you need more light to read or that it's become harder to distinguish black from blue. Although these dry macular degeneration symptoms can accompany many aging eyes, they are also early signs of macular degeneration.
The first sign of macular degeneration may be detected by your eye specialist during a routine eye exam.
Drusen are small yellow or off white deposits under the retina. Alone they do not cause macular degeneration, but an increase in the size and number of drusen does increase your risk for age-related macular degeneration (AMD)
Other symptoms are:
√ Difficulty seeing things at a distance,
√ Blurred vision and
√ Loss of detailed or sharp vision
√ Difficulty distinguishing between colors that are similar such as dark blue and black
√ Loss of contrast vision
√ Difficulty seeing detail
√ Difficulty recognizing a face because there is a blurred spot or a smudge in the center of your vision
The development of age related macular degeneration is silent and painless which is why it is important to have regular eye exams.
It is very important that yearly eye exams are performed to detect any early symptoms of macular degeneration or to begin treatment if any macular degenerative disease is detected.
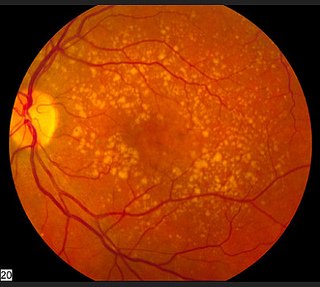
Macular Drusen
The first clue your ophthalmologist will have that you have dry AMD is the presence of drusen which are yellowish deposits of lipids (waste material) in the macula (the center of the retina). You can see the yellow specks in this picture from the National Eye Institute.
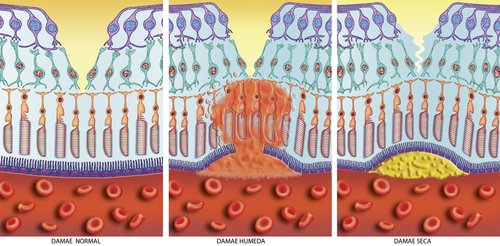
There are actually two types of drusen - hard drusen which is less harmful and soft drusen which is an indicator of macular degeneration. The picture in the far right shows how the build up causes the normally flat layers of the retina (the picture in the far left) to raise which in turn distorts vision.
Find out what questions to ask your doctor about this early sign of age related macular degeneration:
Early Macular Degeneration
Dry AMD has three stages and each eye may be in a different stage of AMD. It is believed that all macular degeneration begins with the dry form.
People with early AMD have either several small, soft drusen or a few medium-sized drusen.
At this stage, there are no symptoms and no vision loss. If persons are diagnosed with the early form of dry AMD, usually they are told that nothing can be done to reverse their condition or to halt its progression.
Patients are often encouraged to:
√ Take vitamins for macular degeneration
√ Wear a visor or hat
√ Test themselves frequently using a macular degeneration grid or Amsler Grid
√ Protect their eyes from the sun by wearing 100% UV protection
sunglasses,
√ Follow a
macular degeneration diet
Find out more about what it means to have this stage of AMD:
Early Macular Degeneration
Intermediate Dry AMD
People with intermediate AMD have:
√ either many medium-sized drusen or one or more large,irregular shaped drusen (called soft drusen)
√ may have a blurry spot or blurred central vision called a central scotoma in the center of their vision
√ may have a distortion of images in the central part of their vision
√ require more light for reading and other tasks
√ higher contrast is needed to see things
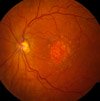
Geographic Atrophy - Advanced Dry AMD
Geographic atrophy is the third stage of dry macular degeneration.
In addition to drusen, , people with advanced AMD, or geographic atrophy, have a breakdown of light-sensitive cells and nearby tissue in the central retinal area. This breakdown can cause a blurred spot in the center of your vision. Over time, the blurred spot may get bigger and darker, taking more of your central vision. You may have difficulty reading or recognizing faces until they are very close to you.
Macular degeneration is a progressive disease. The progression is different for each person. The progression can be very slow and take many years or it can turn quickly and develop into wet AMD or geographic atrophy. Click here to read more about late-stage dry macular degeneration better known as geographic atrophy:
Dry AMD can turn into wet macular degeneration in 10-15% of those with dry AMD.
Find out the difference between dry and wet macular degeneration from the American Academy of Ophthalmology:
What's the Difference between Dry and Wet AMD
Macular Degeneration Vitamins
High concentrations of natural carotenoids can help protect the lens and the retina of aging eyes.
Dr Mahmood Piraee is the co-founder of Persavita Ltd. (Cambridge, UK), and is qualified as a pharmacist (Shahid Beheshti University of Medical Sciences, Tehran). He subsequently completed a PhD in molecular and cellular biology (Dalhousie University, Halifax, Canada). He shares with us how eye health nutrients may protect the retina and the lens of the eye from damage caused by oxidation.
Dry Macular Degeneration Treatment
Although the dry form is the most common type of AMD, traditional medicine has little to offer. There are several dry AMD clinical trials going on right now to find ways of intervening or to slow it's progression.
Complementary or alternative medicine treatments include herbs, microcurrent stimulation or acupuncture. At this time there are no FDA approved medications for the treatment of dry macular degeneration. However, that doesn't mean that there aren't things you can do to help support the health of your eyes and your body.
Plenty of research confirms the role of diet, lifestyle choices and nutritional supplements in the progression or lack of progression of this eye disease.
Find out what factors can influence the health of the macula by clicking here: Dry AMD Treatment
Dry Macular Degeneration Prognosis
How fast will my dry macular degeneration progress? Can dry AMD turn into wet AMD? Will my eye sight get worse? These are all great questions.
The progression of AMD is different for everybody and different for each eye. Some people with dry macular degeneration may hardly notice any changes in their vision and it can remain that way for many years. Others may suddenly wake up one morning shocked to find that they have lost their central vision.
The uncertainty of not knowing how or when one's macular degeneration will progress is a source of stress for many people. Learning about this retinal disease through books about macular degeneration can help you with lifestyle changes to help slow the progression of AMD or teach you how others are finding ways to cope and stay independent even with vision loss.
Find out what factors can influence the progression of this retinal disease and hear from others with age related macular degeneration share their story:
Macular Degeneration Prognosis
Go from Dry Macular Degeneration to WebRN Macular Degeneration Home
√ Prevention of Macular Degeneration?
√ Tips for Daily Living?
√ Food Suggestions for a Macular Degeneration Diet?
√ Ideas on Visual Aids to Maximize your Sight?
If you said "yes" to any of the above, sign up for the monthly Macular Degeneration News.