Geographic Atrophy
Advanced Dry Macular Degeneration Symptoms and Research
Geographic atrophy (GA) is considered late stage dry age related macular degeneration.
Atrophy is the the wasting away or the deterioration of cells or systems. You usually hear these words in association with muscle atrophy - which is the loss of muscles due to lack of use or exercise.
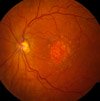
However, in the case of our macula - the center of the retina responsible for our straight ahead, sharp detailed vision - the cone cells waste away due to lack of oxygen and nutrients and the inability to get rid of built up waste products. Here is a picture of geographic atrophy from the National Eye Institute.
Credit: National Eye Institute, NIH Ref#: EDA23
Advanced Stage of Dry Macular Degeneration
Dry or atrophic macular degeneration (AMD) is identified by three macular degeneration stages. AMD is a progressive eye disease. Each eye may be in a different stage.
Advanced AMD is the last stage of this progressive retinal disease. This late stage, or advanced AMD, is identified by:
√ Circular patches of partial or complete depigmentation of one of the layers of the retina, the retinal pigment epithelium. (RPE).
√ Usually there is exposure of another layer of tissue, the choroidal blood vessels
These changes in the RPE may stabilize or they may progress. "Degeneration of RPE cells leads to the death of rods and codes. What an eye doctor sees in a dilated eye exam in a patient with GA is like a continent of atrophy surrounded by a sea of retina.
The continent appears different from the surrounding retina because of the loss of the pigmented RPE cells.
Areas of geographic atrophy look whiter than the surrounding retina."
Janet Sunness, M. D. director of Hoover Low Vision Services at the Greater Baltimore Medical Center
The area of atrophy will determine the amount of vision loss.
Geographic Atrophy Symptoms
Blind Spot in the Center of One's Vision
Dr. Michael A. Samuel, a retina specialist and author of Macular Degeneration: A Complete Guide for Patients and Their Families,describes geographic atrophy as, "the end stage of dry AMD, where central vision is lost."
This loss of our straight ahead vision is called a central scotoma - it is a blurred, gray or black spot that appears in the center of one's vision.
Other vision changes involve:
1. Reading slower
The speed of reading drops as GA progresses
2. Require more light
Difficulty seeing in low light conditions
3. Require more magnification
"Forty percent of people with GA require print to be twice as large after 2 years of worsening," says Dr. Sunness.
Risk Factors for Geographic Atrophy
The most common risk factors for geographic atrophy include:
1. Chronic inflammation
3. Genetics
4. Age
Dr. Sunness states that "more than half of patients with GA in one eye develop GA in the other eye."
You can find out more from this very thorough article on Geographic Atrophy written by the Angiogenesis Foundation.
Geographic Atrophy and Emerging Therapeutic Targets
Geographic Atrophy Clinical Trials
There are several AMD clinical trials that are investigating new ways to treat advanced dry age related macular degeneration.
1) Mitochondria and Macular Degeneration
"Mitochondrial dysfunction is believed to be a significant contributor to the progression of dry AMD, making the mitochondrial network an attractive target to improve retinal function and mitigate disease burdens in this patient population," explains Stealth Biotherapeutics.
Stealth BioTherapeutics Corp is a biopharmaceutical company that is focused on treating diseases that involve mitochondrial dysfunction.
The ReClaim-2 clinical trial has 40 study sites and will include approximately 180 participants who have at least one eye with dry AMD with geographic atrophy. It is a randomized, double-masked, placebo controlled study that is evaluating the safety, efficacy and pharmacokinetics of elamipretide in subjects with Age-Related Macular Degeneration with non-central Geographic Atrophy.
"It is exciting to confirm the relationship between elamipretide-mediated improvements in visual function and mitochondrial health in patients suffering from progressive vision loss due to GA," said Chief Executive Officer Reenie McCarthy. "These data support our confidence in our ReCLAIM-2 trial design, which focuses on the earlier stages of GA before irreversible mitochondrial and RPE damage is thought to have occurred. We are encouraged to learn that intervening at this stage of the disease may not only slow disease progression, but also restore visual function and visual quality of life to patients suffering from this life-limiting disease."
ReCLAIM-2 Study to Evaluate Safety,Efficacy & Pharmacokinetics of Elamipretide in Subjects With AMD With Non-central GA (ReCLAIM-2)
2) Zimura Intravitreous Injections
This Phase 2B clinical trial was a randomized, double-masked, sham-controlled study to determine the safety and efficacy of intravitreous injections of Zimura. With 78 study locations in the U.S. and globally, 286 patients with geographic atrophy due to dry age related macular degeneration participated in this research. Patients were assigned to different groups who received either a sham treatment or varying doses of the active ingredient.
The ability to potentially slow the process of retinal cell degeneration and reduce the rate of geographic atrophy growth in those with dry age-related macular degeneration was shown by these preliminary results:
Ocular Surgery News reported on October 28, 2019
"Patients who received Zimura (avacincaptad pegol) 2 mg experienced a mean reduction in geographic atrophy growth of 27.38% at 12 months compared with the sham group (P = .0072). Those who received Zimura 4 mg had a mean reduction of 27.81% compared with sham (P = .0051)."
Updated progress in the Phase 3 GATHER2 study from Ophthalmology Times September 6, 2022
"Iveric Bio Inc. today announced positive topline results from GATHER2, the company’s second Phase 3 clinical trial of avacincaptad pegol (Zimure), a novel investigational complement C5 inhibitor, for the treatment of geographic atrophy (GA). According to the company, GATHER2 met its prespecified primary endpoint of mean rate of growth (slope) in GA area at 12 months with statistical significance and a favorable safety profile."
Zimura in Subjects With Geographic Atrophy Secondary to Dry Age-Related Macular Degeneration
3) Adult Stem Cell Based Therapeutic Approach
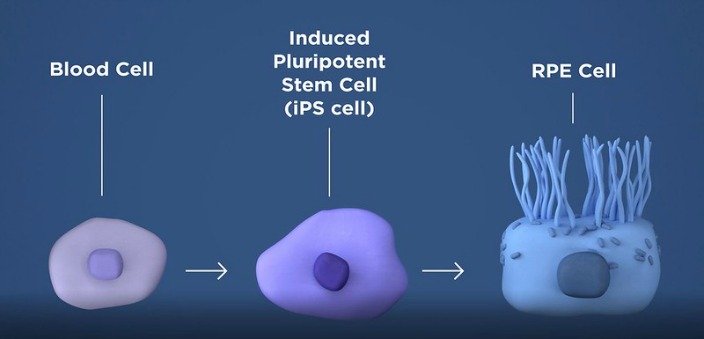
A January 16, 2019 news release from the National Institute of Health reported that "Ten weeks after the human iPSC-derived RPE patches were implanted in the animals' retinas, imaging studies confirmed that the lab-made cells had integrated within the animal retina."
Taking a patient's own blood cells, researchers are able to convert the blood cells into induced pluripotent stem cells (iPSC). This type of cell can then be "induced" to become any type of cell. By programming the stem cells to become retinal pigment epithelial (RPE) cells and transplanting the the patch of healthy RPE cells between the patient's RPE and photoreceptors, the layer of photoreceptor cells can now be supported to prevent any further death of the rods and cones. Since the patient's own blood cells are used, the chance of rejection of the RPE transplant is minimized.
“The protocol, which prevented blindness in animal models, is the first clinical trial in the U.S. to use replacement tissues from patient-derived induced pluripotent stem cells (iPSC),” said Kapil Bharti, Ph.D., a senior investigator and head of the NEI Ocular and Stem Cell Translational Research Section.
This dry macular degeneration treatment breakthrough is now in a Phase I/IIa clinical trial being conducted at the National Institutes of Health Clinical Center in Bethesda, Maryland.
You can find out more here...
4) Antibiotics to Treat Geographic Atrophy
The National Eye Institute is looking to see if a common antibiotic, minocycline, will help those with geographic atrophy. (GA).
Dry macular degeneration has a slower progression of central vision loss due to the degeneration and loss of the retinal pigment epithelial (RPE) cells and photoreceptors in the macula.
According to the description from ClinicalTrials.gov, "While the etiology of GA is not completely understood, inflammatory processes involving the activation of resident immune cells of the retina called microglia is likely to contribute. Minocycline inhibits the activation of microglia which produce inflammatory factors implicated in GA development. The objective of this study is to investigate the safety and possible efficacy of oral minocycline in patients with GA."
Forty participants who have GA in one or both eyes will be enrolled in the study. A 100 mg dose of minocycline will be taken twice daily for 36 months. To find out more about this study visit:
Go from Geographic Atrophy to Dry Macular Degeneration
Go from Geographic Atrophy to Macular Degeneration
√ Prevention of Macular Degeneration?
√ Tips for Daily Living?
√ Food Suggestions for a Macular Degeneration Diet?
√ Ideas on Visual Aids to Maximize your Sight?
If you said "yes" to any of the above, sign up for the monthly Macular Degeneration News.